1 Context and Positioning
In this chapter, we give the essential background for understanding the principles of muscle contraction. Some elements of this presentation will be refined in appropriate subsequent chapters.
1.1 Muscle contraction in brief
1.1.1 The contractile structure
The human body contains three types of muscles: the skeletal muscles, responsible for motion, the cardiac muscle responsible for the blood circulation, and the smooths muscles found essentially in the digestive apparatus. This manuscript focuses on the group of striated muscles which contains the skeletal and cardiac muscles.
The striated muscle tissue consists in a hierarchy of bundled fibers, with the highest level corresponding to the tissue itself, see Figure 1.1. The skeletal muscles contain fascicles (diameter
In the skeletal muscle, the myocytes, also called muscle fibers, are the centimeter-long fibrilar cells responsible for the contraction. In the cardiac muscle, the contractile cells (cardiomyocytes) are shorter (50-100 μm ) and often branched. The cytoplasm of myocytes and cardiomyocytes contains mostly a parallel arrangement of 2 μm diameter myofibrils, in addition to the classical cell organelles (nucleus, mitochondria etc.).
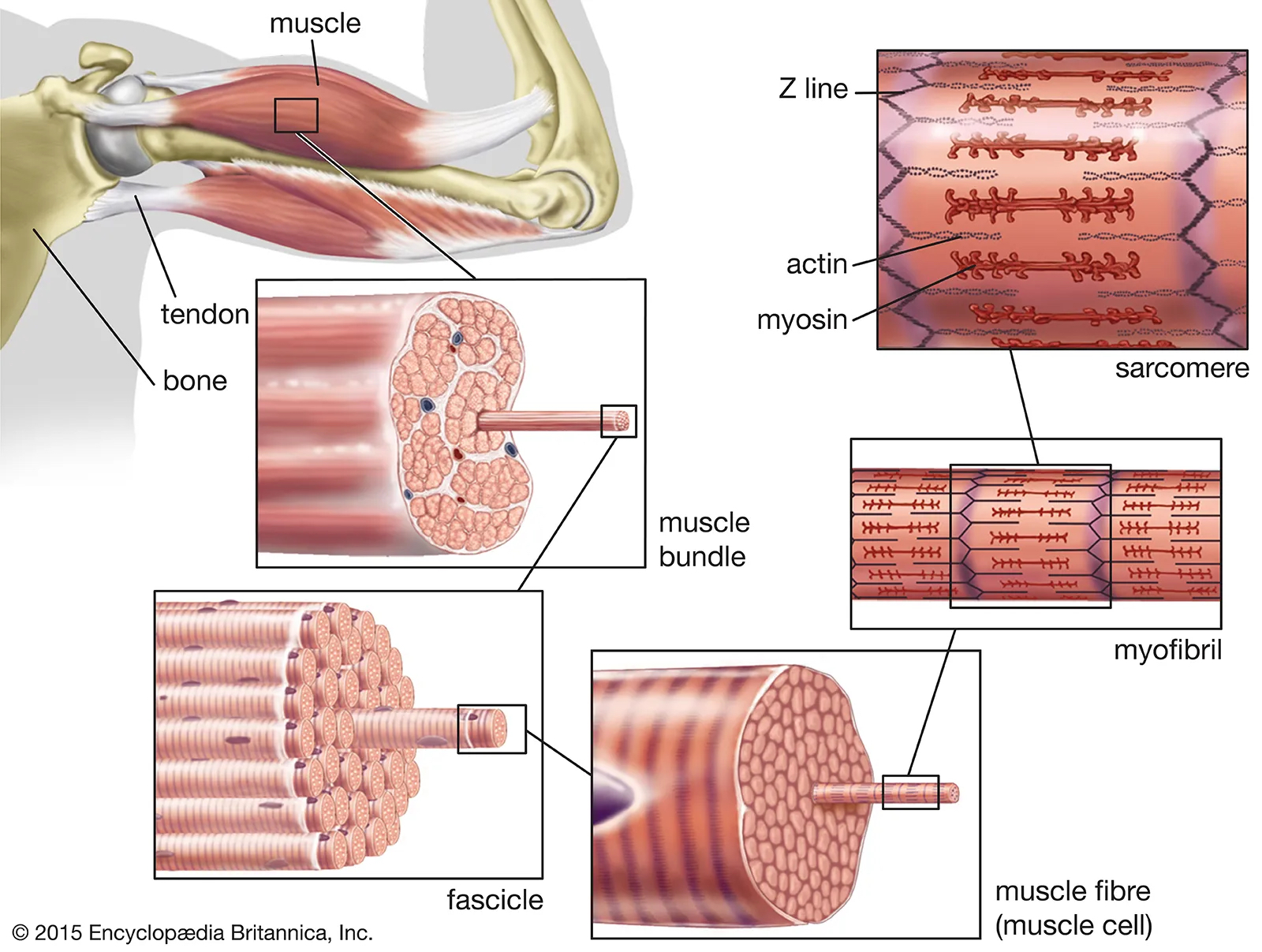
The core of the contractile machinery is embedded in the myofibrils, see Figure 1.2. It consists of a highly regular longitudinal succession of
In the cross-section, actin and myosin filament form a crystalline hexagonal lattice running parallel to the direction of the fiber. The unit cell of this lattice thus contains one thick filament and two thin filaments. When the muscle contracts, the myosin proteins that constitute the thick filaments induce a relative sliding of the surrouding actin filaments that results in the shortening of the sarcomeres. This active shortening is due to the metabolic activity of the myosin proteins which act as molecular motors.
The contractile structure also involves other cytoskeletal proteins (see M-lines, Z-disks and titin in Figure 1.2) that will be presented in more details in Section 5.1.1. We will now focus on the molecular mechanism of force generation by the molecular motors.
1.1.2 Molecular mechanism of contraction
The thick filament is a bundle of 600 myosin proteins connected by their tails. They form into two antagonists groups of 300 individuals each, see Figure 1.2. In the rest of this manuscript, we will call a contractile unit an effective bundle of 300 molecular motors constituting a half muosin filament interacting with its surrounding actin filaments. Given the relative proportion of myosin and actin filament, such contractile unit can be effectivement represented as a single myosin filament interacting with two actin filaments.
A single myosin protein has a pair of heads that points radially, from the myosin filament towards the surrounding thin filaments. It is the cyclic interaction between myosin heads and actin that produces the active force necessary to shorten the sarcomeres. The actin-myosin interaction cycle is referred to as the Lymn and Taylor cycle.1 see Figure 1.3.
1 “Mechanism of Adenosine Triphosphate Hydrolysis by Actomyosin,” 1971. Here we present the classical version contains four steps. Since their fundamental 1971 publication, numerous tudies on the molecular mechanism of force generation have contributed to the refinement of this cycle. See for instance the review by Houdusse and Sweeney (“How Myosin Generates Force on Actin Filaments,” 2016).
When the muscle contraction is activated2 the myosin heads can attach to specific actin binding sites regularly positioned along the actin filaments (step
2 see Chapter 4 for more details about the activation process.
3 Huxley and Simmons, “Proposed mechanism of force generation in striated muscle,” 1971; Rayment et al., “Structure of the actin-myosin complex and its implications for muscle contraction,” 1993; Rayment et al., “Three-dimensional structure of myosin subfragment-1: A molecular motor,” 1993.
4 The exact sequence of events leading to the departure of Pi from the myosin active site and their relationship with the power-stroke not fully resolved. See Debold, “Recent insights into the relative timing of myosin’s powerstroke and release of phosphate,” 2021. For more details about the molecular mechanisms of the enzymatic activity of myosin we refer to Houdusse and Sweeney (“How Myosin Generates Force on Actin Filaments,” 2016).
After the working stroke has been executed, the myosin detaches from actin (step
The collective actin-myosin interactions, which generate antagonistically oriented working strokes inside each sarcomere, is ultimately responsible for the macroscopic contraction of the muscle fibers.
1.2 Why study muscle contraction? The example of cardiomyopathies
Pathological alterations of the complex contractile apparatus are involved in the development of genetic cardiomyopathies, which, in the most severe cases, lead to Acute Heart Failure (AHF) and sudden death.
The causes of AHF are diverse, which makes it particularly difficult to treat.5 The most prevalent genetic cardiomyopathy degenerating into AHF is the Hypertrophic Cardiomyopathy (HCM), affecting 1 individual per 500.6 This pathology is often associated with mutations of the myosin protein that result in an increase of the tissue contractility.7 Another severe genetic cardiomyopathy leading to AHF is the Dilated Cardiomyopathy (DCM) which accompanies the deterioration of the mechanical properties of the M-lines Z-lines and titin, resulting in the loss of register of the contractile units inside the fibrils (see Figure 1.2), and abnormal inflation of the tissue over time.8 The mechanisms underlying HCM and DCM are not well understood. The connection between protein alterations at various biological levels and the resulting observable effects on heart function has not been clearly established.
5 Rossignol et al., “Heart failure drug treatment,” 2019; George et al., “Novel drug targets in clinical development for heart failure.” 2014.
8 Hinson et al., “Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy,” 2015; Lange et al., “The M-band: The underestimated part of the sarcomere,” 2019; Gerull et al., “Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy,” 2002; Granzier et al., “Titin: Physiological Function and Role in Cardiomyopathy and Failure,” 2005.
9 reviewed in Day, Tardiff, and Ostap, “Myosin modulators: Emerging approaches for the treatment of cardiomyopathies and heart failure,” 2022; RabieeRad et al., “Novel Treatments of Hypertrophic Cardiomyopathy in GDMT for Heart Failure: A State-of-art Review,” 2023; Houdusse et al., “Small molecules modulating force production: A promising strategy to treat myosin-associated diseases,” 2024.
10 As per 2025, the FDA has not approved the use of Omecamtiv Mecarbil as a treatment of heart failure.
A promising therapeutic approach in AHF, HCM, DCM and other cardiomyopathies is the use of small effector molecules, such as Omecamtiv Mecarbil and Mavacamten, that specifically modulate myosin activity.9 For instance, the clinical trials have shown that cardiac function of patients with severe cardiomyopathies can be improved within minutes of inoculation with Omecamtiv Mecarbil.10 It is worth noticing that Mavacamten has recently been approved by the U.S. Food and Drug Administration.11
Despite the proven clinical efficacy of these molecules, there remains an unclear understanding of their impact on the contraction process, potentially complicating the adjustment of prescriptions. For this reason, and because of their promising impact on deseases, these drugs are under intense experimental scrutiny at the structural, cellular and organ (or body) scales.12
1.3 Some motivations
Between the nano- and the macro-scale, a full understanding of how the drugs or pathologic mutation modify the contraction process is still lacking, even at the most basic mechanical level. Practitioners could leverage this understanding in their clinical decisions. For this reason, mutations leading to cardiomyopathies and the associated treatments are considered to be priority research subjects.13
Existing experimental studies provide valuable information on the effect of mutation and drugs at specific scales of the tissues. The rationale of research is to leverage this knowledge to design a unified modeling framework to emulate the genotype to phenotype relationship in healthy and diseased muscle tissue. Furthermore, the knowledge of the mechanical and physiological pathways explaining this relationship, would open the possibility to engineer small protein effectors based on the simulation of their desired macroscopic effect.
Another potential outcome of the research efforts in understanding the physical principles governing muscle contraction is to design artificial actuators leveraging similar principles. As an example, we may consider electromechanical actuators being used in machines or vehicles. In some applications, in partiular aircraft engineering, critical functions can be impaired by the malfunction of a single actuator. In such situations, critical motions are often ensured by redundant actuators for savety reasons. However, in some cases, redundancy is not permitted, either because of steric hindering (e.g. for helicopters) or because of weight cost (e.g. for spatial application). Muscle resilience originates from its multiscale structure and multileveled neuronal command. The structure and command strategies may be advantageously mimicked to create a new kind of resilient actuator. Moreover, recent advancements in nanomachines constructed from purified muscle proteins suggest the potential design of actuators assembled from genuine proteins.14
In summary, the goal of our research is to provide models of the contraction at different scales that can be used
- to understand the specific impact of pathological mutations and drugs on the mechanisms of contraction,
- to design artificial devices that mimicks the outstanding properties of muscle cells and tissue.
1.4 Four scales
We view the modeling framework as the integration of four projects, each being specific to one of the following scales:
- the nanoscale,
- the microscale,
- the mesoscale,
- the macroscale.
In the following paragraphs we introduce briefly the context of each scale. More details are available in the dedicated chapters.
1.4.1 Nanoscale: the molecular motor
The typical size of a protein is just a few nanometers. At this scale, the objective is to understand and model:
- The mechanics of allosteric enzymatic activity in myosin, specifically its ability to catalyze ATP hydrolysis within an active site of the molecule, and use this reaction to trigger a significant conformational change at a distant site within the molecule.
- The physical principles underlying the interaction between a single myosin protein and an actin filament.
Our goal at the nanoscale is to provide a physiologically relevant dynamical model of the actin-myosin interaction, based on a minimal set of descriptive variables.
1.4.2 Microscale: the contractile unit
We define a contractile unit a bundle of molecular motors sharing the same myosin filament backbone and interacting with the surrounding actin filaments. Considering the stoechiometry between the two types of filaments, the contractile unit comprises one thick filament and two thin filaments. At this scale, the objective is to understand how the molecular motors cooperate. The cooperative mechanisms emerge from the elastic coupling that is mediated by the filament themselves and by the titin protein.15 Such coupling plays a fundamental role not only in the basic force production but also in the activation and regulation of this process.16
Our goal is to understand how the connections between the molecular motors at the scale of a prototypical contractile unit affect their collective functionning, in particular the force production and its regulation.
1.4.3 Mesoscale: the sarcomere and the myofibril
Stacks of contracile units are arranged parallel to form sarcomeres which are, in turn, assembled in series within muscle fibrils and fibers, see Figure 1.2. It is the synchronized contraction of all activated contractile units that enables the macroscopic shortening of the muscle fibers.
The mechanical coupling between the contractile units is facilitated by scaffolding structures (such as Z-lines, M-lines, and titin), which maintain the entire hierarchy in alignment and preserve its integrity. These structures also play a fundamental role in the regulation of the contraction.17 Finally, it is now clear that mutations affecting the mechanical properties of the M-lines, Z-disks and titin cytoskeletal proteins that constitute this elastic network, are involved in the development of cardiomyopathies, potentially via an alteration of their ability to maintain the sarcomeres in register.18
18 Hinson et al., “Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy,” 2015; Lange et al., “The M-band: The underestimated part of the sarcomere,” 2019; Gerull et al., “Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy,” 2002; Granzier et al., “Titin: Physiological Function and Role in Cardiomyopathy and Failure,” 2005; Herwig et al., “Modulation of Titin-Based Stiffness in Hypertrophic Cardiomyopathy via Protein Kinase D,” 2020; Wadmore, Azad, and Gehmlich, “The Role of Z-disc Proteins in Myopathy and Cardiomyopathy,” 2021.
Finally, the cardiac tissue being a composite material with active stress fibers embedded in the extracellular matrix, the mesoscale structure is also characterized by the orientation of the fibers within the tissue. Microscope observations show that the fiber orientation distribution in healthy and pathological tissues may greatly differ.19
Our goals at the mesoscale are to
- provide a physiologically valid model of the network of sarcomeric structural proteins and study how its properties affect the collective functioning of contractile units,
- formulate a homogenized macroscopic behavior law of the tissue that takes into account its composite microstructure.
1.4.4 Macroscale: muscle tissue
The macroscale is the scale of the contracting tissue itself. Macroscopic data include the typical continnum mechanics fields of displacement, deformation, stresses etc. In the case of the heart they also include the internal pressure, the ventricules or atria volumes and the blood flow rates in and out of the cavities.
The challenge is to provide a model that can reproduce these macroscopic observables using ingredients reminiscent of the actual physiological process. Since these processses are complex, the models may involve many parameters that end up to be difficult to calibrate. Another difficulty lies in the numerical cost of these models which can impair their use in clinical contexts.
1.5 Positionning
In this manuscript, we will position our work mainly with respect to research on the heart contraction in health and disease. However, the models that will be presented can be used with other types of muscle as well.
1.5.1 Experimental research
Muscle contraction mechanisms can be studied experimentally at all scales.
- Nanoscale, molecular motors
3D Molecular structures of the various myosin conformations involved in the contraction cycle can be resolved from X-Ray crystallography and/or Cryogenic Electron Microscopy, both in control and in the presence of mutations or small effectors, giving insight into how they could influence the molecular mechanism of force generation.20 These crystallographic structures can be viewed as high resolution snapshots of the protein in different conformations.21 It is then possible to use molecular dynamics simulation to get insight into the force generation mechanism over short timescales. The properties of isolated molecular motors can also be probed using mechanical tests in optical tweezers.22
22 Arbore et al., “Probing force in living cells with optical tweezers: From single-molecule mechanics to cell mechanotransduction,” 2019; Woody et al., “Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke,” 2018; Woody et al., “Single molecule mechanics resolves the earliest events in force generation by cardiac myosin,” 2019; Yanagida et al., “Single molecule analysis of the actomyosin motor,” 2000.
Finally, taking advantage of the crystalline nature of the muscle myofibrils, X-ray diffraction can be used in-situ to probe nanoscale structural change in real time, while performing a mechanical test on a macroscopic sample.23 In the latter type of experiment, the average behavior of a motor can be monitored with nanometer resolution while it interacts with other motors in near to physiological conditions.
- Microscale, contractile units
Interactions between molecular motors within contractile units can be observed in artificial preparations.24 In in-vitro motility assays for instance, large groups of myosin motors are fixed on a plane surface and moving actin filament put on to of them.25 Contractile units involving a controled number of motors can also be manipulated in vitro. Recently the team lead by Dr. P. Bianco from the PhysioLab (University of Florence, Italy) has succeeded in reconstructing a minimal functional contractile unit out of purified actin and myosin proteins, able to reproduce the performance of the functional unit of the muscle.26 These experimental setups provide ideal platforms for testing the basic effect of new drugs and for designing artificial biomimetic devices.
25 Warshaw, “The In Vitro Motility Assay: A Window Into the Myosin Molecular Motor,” 1996; Holzbaur and Goldman, “Coordination of molecular motors: From in vitro assays to intracellular dynamics,” 2010.
26 Pertici et al., “A myosin II nanomachine mimicking the striated muscle,” 2018; Pertici et al., “A Myosin II-Based Nanomachine Devised for the Study of Ca2+-Dependent Mechanisms of Muscle Regulation,” 2020; Buonfiglio et al., “Force and kinetics of fast and slow muscle myosin determined with a synthetic sarcomere–like nanomachine,” 2024, see also Kaya et al., “Coordinated force generation of skeletal myosins in myofilaments through motor coupling,” 2017; Cheng, Leite, and Rassier, “The load dependence and the force-velocity relation in intact myosin filaments from skeletal and smooth muscles,” 2020; Hwang et al., “A reverse stroke characterizes the force generation of cardiac myofilaments, leading to an understanding of heart function,” 2021.
- Mesoscale, Inter-sarcomere dynamics and regulation
Over the past two decades, experimental studies at the scale of single myofibrils or fibers have revealed the existence of non-uniformities (non-affine behavior) of the sarcomere lengths during contraction. The direct observation can come from single fibrils tested in optical tweezer setups or from single cell preparations using fast confocal microscopy. Recent reviews of these studies report on the important role played by the elastic network that connects the sarcomeres together in tailoring these non-uniformities.27
27 Leite and Rassier, “Sarcomere length non-uniformity and force regulation in myofibrils and sarcomeres,” 2020; Linke, “Stretching the story of titin and muscle function,” 2023; Herzog and Schappacher-Tilp, “Molecular mechanisms of muscle contraction: A historical perspective,” 2023.
28 Ait-Mou et al., “Titin strain contributes to the Frank–Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins,” 2016; Linke and Krüger, “The Giant Protein Titin as an Integrator of Myocyte Signaling Pathways,” 2010; Caremani et al., “The force of the myosin motor sets cooperativity in thin filament activation of skeletal muscles,” 2022; Brunello and Fusi, “Regulating Striated Muscle Contraction: Through Thick and Thin,” 2024.
It is also hypothesized that these sarcomeric proteins participate in fundamental regulation pathways involving mechanical feedback-loops: the force transmitted via the elastic scaffold may enhance contractile units’ activation.28 Recording myosin based reflexions gives access to the footprint of titin modulation of the regulatory state of the myosin filament. This approach allowed understanding of how the titin react to activation signals and how it can activate the molecular motors.29
Finally, the distribution of fiber orientation in the tissue can be quantified post-mortem using standard microscopy or using diffusion-weighted MR imaging (DW-MRI).30
30 Hoshino et al. “Myocardial fiber diameter and regional distribution in the ventricular wall of normal adult hearts, hypertensive hearts and hearts with hypertrophic cardiomyopathy,” 1983; Damon et al. “Skeletal muscle diffusion tensor-MRI fiber tracking: Rationale, data acquisition and analysis methods, applications and future directions,” 2017
- Macroscale, experiments on intact trabeculae
The majority of experimental data are obtained from mechanical tests performed on multicellular preparations. These tests allow studying the effects of drugs and mutations on the tissue contraction. For skeletal muscles, in situ experiments are performed either on single fibers or directly on intact muscle preparations. For the heart muscle, a large body of experimental data is obtained from mechanical tests performed on multicellular preparations—typically the pillar-shaped cardiac trabecula31—isolated from ventricles.32
31 the trabeculae muscle (or trabeculae carneae) are muscular columns that project from the inner surface of the right and left ventricles. They may form simple ridges, or be fixed at both extremities. Papillary muscles are examples of trabeculae that holds the tendinous chords holding the cusps of the valves. Trabeculae muscles are used in experiments because they are essentially one dimensional objects with aligned fibers.
32 de Tombe and ter Keurs, “Force and velocity of sarcomere shortening in trabeculae from rat heart. Effects of temperature.” 1990; Ait Mou et al., “Altered myofilament structure and function in dogs with Duchenne muscular dystrophy cardiomyopathy,” 2018; Caremani et al., “Size and speed of the working stroke of cardiac myosin in situ,” 2016; Pinzauti et al., “The force and stiffness of myosin motors in the isometric twitch of a cardiac trabecula and the effect of the extracellular calcium concentration,” 2018.
The mechanisms of contraction can then be tested in a wide range of conditions by varying the content of the bathing solution, the temperature and the mechanical loading. As mentioned above, this type of experiment, can be coupled with nanometer resolution X-ray diffraction measurements.33
At the scale of the organ, macroscopic deformation maps can be recovered from tagged cine MRI,34 and catheter pressure probes inserted in the heart allow recording pressure-volume loops.35
35 Bastos et al., “Invasive left ventricle pressure–volume analysis: Overview and practical clinical implications,” 2020; Protti et al., “Looking Back, Going Forward: Understanding Cardiac Pathophysiology from Pressure–Volume Loops,” 2024; Foëx and Leone, “Pressure-volume loops: A dynamic approach to the assessment of ventricular function,” 1994.
1.5.2 Mechanical models and heart simulations
The interest of having a physiologically relevant model at organ-scale is obvious: it allows simulating the contraction and have access to various indicators that can be compared to clinical data on the one hand but also estimates of internal parameters that are not directly accessible by macroscale measurements, such as internal stresses.
To link these internal parameters to the macroscopic observables, comprehensive models of the heart covering all physiological aspects of its functioning are already available. International research consortia are already using this kind of models to study cardiomyopathies and their treatments, see for instance the work of the SilicoFCM consortium or the MATHCARD project.
In the last decade, computer methods have been developed to simulate realistic muscle behavior, especially for the heart.36 Essential underlying model elements are the force generation by molecular acto-myosin motors, the activation processes regulating contraction, the perfusion mechanisms (for the heart), and the passive viscoelastic properties of the tissue.
36 Chabiniok et al., “Multiphysics and multiscale modelling, datamodel fusion and integration of organ physiology in the clinic,” 2016; Stojanovic et al., “Multi-scale striated muscle contraction model linking sarcomere length-dependent cross-bridge kinetics to macroscopic deformation,” 2019; Regazzoni, Dedè, and Quarteroni, “Biophysically detailed mathematical models of multiscale cardiac active mechanics,” 2020; Sugiura et al., “Multi-scale simulations of cardiac electrophysiology and mechanics using the University of Tokyo heart simulator,” 2012.
All the existing approaches share similar background based on three essential assumptions.
- The molecular motors’ behavior can be represented as a jump process between a set of states characterizing the conformation of the myosin motors, its attachment to actin (attached or detached) and its biochemical state (ATP hydrolysis stage within the active site). The transition rates between these states are dependent on conformational and positional mechanical degrees of freedom. Hence, the designation chemical-mechanical models.
- The bulk density of motors within the tissue is sufficiently large for a mean-field description to be valid. The motors population dynamics is then governed by a (potentially large) system of Partial Differential Equations on the probability distributions characterizing each “chemical state” of the motors. Mathematical simplifications (surrogate models) and associated numerical schemes have been developed to make this system of governing equations suitable for organ-scale finite elements simulations37
- The equations describing the population of motors are directly coupled to the equilibrium laws of continuum mechanics, using rheological lumped elements that represent the contribution of cytoskeletal structural proteins to the passive viscoelastic properties of the tissue. It is usually assumed that active fibers are homogenous and that a unique fiber direction can be defined at each material point.
37 Kimmig and Caruel, “Hierarchical modeling of force generation in cardiac muscle,” 2020; Milićević et al., “Huxley muscle model surrogates for high-speed multi-scale simulations of cardiac contraction,” 2022; Regazzoni et al., “A machine learning method for real-time numerical simulations of cardiac electromechanics,” 2022.
In view of these modeling hypotheses, we can point several limitations of the existing frameworks.
- The chemical-mechanical model of the molecular motor functioning is in fact an asymptotic represenation of a high-dimensional stochastic system describing the motion of interconnected atoms or amino-acids. There is no systematic derivation such asymptotic model reduction, which is in fact valid only if the so-called states represent deep enough energy wells. There is also no formal link between the chemical-mechanical model and the underlying structural mechanisms and conformational changes.
- The mean-field assumption made to describe a large population of molecular motors neglects the mechanical interactions that can exist at the level of single contractile units, i.e. where a finite size system of molecular motors are coupled via the myofilaments. It also corresponds to an asymptotic limit that would need to be mathematically justified.
- Using the assumption of a direct coupling between the molecular scale processes and the macroscopic mechanical balance laws, current models consider that a muscle fiber can be viewed as a homogenous 1D continuum, without detailed modeling of the cytoskeletal proteins at the mesoscale and without questioning the validity of having a single fiber orientation per material point. However, as mentioned in the previous sections, experiments have shown that the cytoskeletal proteins play a role in maintaining contractile units in register, notably during activation.38. Alteration of their mechanical properties may lead to non-affine deformations which could contribute to the development of cardiomyopathies.39 Direct observation have also shown that in pathological situation, the definition of a clear fiber orientation was not possible.40
For these reasons, there is no truly multiscale framework linking the molecular structural alterations induced by cardiomyopathies and their associated pharmacology, with their consequences on the heart.
In the folowing chapters, we will review the different scales of modeling and present an overview of the current state of research, of our contribution to this research, and sketch further developments.